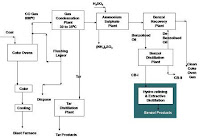
 |
| Production of coal chemicals from coal destruction process: plant block diagram |
coal a solid fuel used as secondary energy source after petroleum fuels and the origin of coal is approximately explained as the same of the petroleum, organic material subjected to high pressures conditions without the presences of oxygen which made their structure to develop hydrocarbon material as coal, when coal is burned in the presence of air components present in it having long range of boiling points(more volatile compounds and heavy molecular weight compounds) will be combusted at different frequencies leaving the flue gas with a lot of noncombustible compounds.
By chemical analysis we can get lots of chemical structured compounds present in it, trapped in a solid form, unlike liquid petroleum it has to be distilled to extract the fractions, but due to it physical property and moreover plants which produce steel and power plants which generate electricity by the coke which is obtained from coal is used as raw material, not these plants all the chemical industries and heavy machinery plants use coke as energy source instead of coal due the chemical compounds present in it, well for the purpose coal is heated in the absence of air up to 1000 to 1200 degree centigrade and all the compounds except carbon rich coke are collected and purified for the chemical compounds,
In a steel manufacturing industries coal is subjected to destructive distillation in a coke oven, for about nearly 24 hr and the gases are collected for extraction of fractions, it is sequence operation
Coke oven gas: gas which is liberated during coking procedure of coal in a coke oven, which consists of valuable by-products, is called coke oven gas.
Separator: the hot gas is cooled in the collection chamber and about 60 percent of tar is condensed and coal particles which are carried over are flushed with a flushing liquid, at separator the liquid and uncondensed gas are separated.
Primary cooler: gas is cooled to 25 to 30-degree centigrade by indirect contact with water in countercurrent flow.
Electrostatic tar precipitator: ETP is used for removal of tar which is in the form of fog present in the coke oven gas from the primary cooler.
Ammonia absorber: coke oven gas contains 3 percent of ammonia and this is reacted with sulphuric acid to form ammonia sulphate fertilizer.
Benzol recovery: benzene, toluene and xylene are in the form of benzol are recovered in this section leaving out clean coke oven gas which is used a fuel for the plant.