A measuring technique used to find the acidic and basic condition of a substance, it mostly indicates the activeness of the substance to react, we can know how stable a compound is, to determine the pH of substance there are easy methods available along with some portable instrument which will used to know the pH of the substance at a instant. The term pH is widely used in the field of chemistry, biochemical and all organic and inorganic concepts. A element hydrogen which has one electron is highly active it tends to react other elements to get a paired electron.
when a data is given for a compound we can calculate theoretical values of pH, pOH, [H+] and [OH-].
By using the relations like =
pH + pOH = 14
Anti log of (-pH) = [H+]
Anti log of (-pOH) = [OH-]
and by definition
pH is defined as negative logarithm of H+ ion and pOH is defined as -ve log of OH- ion
pH = - log [H+]
pOH = - log [OH-]
---------------------------------------------------------------------------------------------------------------------------
Let see an example :Calculate the [H+], [OH-], pH and pOH of 0.001 M HCl solution
we have one H in HCl so the [H+] = molarity of the HCl solution = 0.001 = [H+]
pH = - log([H+]) = -log(0.001) = 3
we have pOH = 14 - pH = 14 - 3 = 11
and now
[OH-] = 10(-[pOH]) = 10^(-11) = 1X10-11
when a data is given for a compound we can calculate theoretical values of pH, pOH, [H+] and [OH-].
By using the relations like =
pH + pOH = 14
Anti log of (-pH) = [H+]
Anti log of (-pOH) = [OH-]
and by definition
pH is defined as negative logarithm of H+ ion and pOH is defined as -ve log of OH- ion
pH = - log [H+]
pOH = - log [OH-]
---------------------------------------------------------------------------------------------------------------------------
Let see an example :Calculate the [H+], [OH-], pH and pOH of 0.001 M HCl solution
we have one H in HCl so the [H+] = molarity of the HCl solution = 0.001 = [H+]
pH = - log([H+]) = -log(0.001) = 3
we have pOH = 14 - pH = 14 - 3 = 11
and now
[OH-] = 10(-[pOH]) = 10^(-11) = 1X10-11
----------------------------------------------------------------------------------------------------------------------------
General:
The pH value of a material is tested by two methods which are (1 ) Electrometrical method and (2) Colorimetrical method. By using electrometric method enough accurate values can be determined but requires special apparatus. The colourimetric method is simple and requires less expensive apparatus, and is sufficiently accurate for general work. It is however subjected to interference by colour, turbidity, high saline content, free chlorine and various oxidants and reductants. In case of dispute, the electrometric method shall be considered as the referee method.
pH scale:
Neutral pH: when pure water dissociates into positive hydrogen(H+) ion and negative Hydroxyl(OH-) ions in equimolar proportional then neutrality is said to exist and water is said to be neutral. It is found that in pure water at 22oC the concentration of H+ and OH- ions equal to 10-7, hence pH value equal to –log (10-7)=7. Hence, pure water is neither acidic nor basic. The acidity of solution increases as pH value falls below 7 with an unlimited value of 0 while the alkalinity of solution increases as pH value rises above 7 with ultimate value of 14. Thus pH scale ranges from 0 to 14. An acid solution of unit strength has pH value 0 and pH value 14 means base solution of unit strength. Note that pH measure only the concentration of H+ ions actually dissociated in a solution and not the total acidity or alkalinity. Due to this reason, the pH value changes with temperature of the liquid. As the water temperature increases the dissociation into H+ and OH- ions increases that result in a decrease in pH value.
Electrometric method: The determination may be made with any pH meter provided with a glass electrode, using instructions from the manufacturer. Express the result to the nearest 0.1 units. Electronic pH measurement system consists of
- Measuring electrode
- Reference electrode
- Potential measuring system
Measuring electrode: A glass electrode is made of a thin glass membrane of special composition that could develop potential proportional to the difference in H+ ion concentration of liquid on either side of the membrane. The glass envelope has pH sensitive glass membrane at the bottom that contains constant pH buffer solution. This electrode is dipped in the measuring solution so that potential is developed at the platinum electrode which is proportional to the pH of the measuring solution. This potential is measured by completing the circuit with the reference electrode.
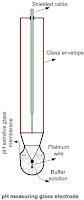 |
| pH measuring glass electrode |
 |
| Reference electrode |
 |
| Potential measuring system for pH determination |
Colourimetric method:
Reagents: A series of indicators and buffer solutions are required for this method. The method of preparation of indicator is given below.
Procedure: Take 100ml of the sample in a hard glass tube and determine the approximate pH by using a universal indicator. Repeat using a solution of the indicator about 1/20 of the volume of the liquid being tested which corresponds to the approximate pH found above.
Compare the colour produced with a series of buffer solutions of known pH each containing the same proportion of the indicator. Report as pH, the pH of that buffer solution which matches with that of the sample, to the nearest 0.1 unit
For determination of pH, we use some indicators to find its magnitude(value) based on colour change
Universal indicator:
To prepare a universal indicator which is used widely and frequently the procedure is, dissolve 0.05g of methyl orange, 0.15g of methyl red, 0.3g of bromothymol blue and 0.35g of phenolphthalein in one-liter alcohol(66 percent) which may be ethanol
And the colour change occurs when two to three drops of this solution is mixed with the sample of which we like to determine pH are as below:
pH
|
Color
|
Up to 3
|
Red
|
4
|
Orange-red
|
5
|
Orange
|
6
|
Yellow
|
7
|
Yellowish-green
|
8
|
Greenish-blue
|
9
|
Blue
|
10
|
Violet
|
11
|
Reddish-violet
|
For accurate determination of pH, a process indication of colour should be referred to the particular range as below.
s.no
|
Name of indicator
|
pH range
|
Colour change
|
Method of preparation
|
1
|
Thymol blue
|
1.2-2.8
|
Red to yellow
|
Tritute 0.1g in 10.75ml of N/50 sodium hydroxide solution and dilute with distilled water to 250ml
|
2
|
Bromophenol blue
|
3.0-4.6
|
Yellow to blue-violet
|
Triturate 0.10g in 7.45ml of N/50 sodium hydroxide solution and dilute with distilled water to 250ml
|
3
|
Bromocresol green
|
3.8-5.4
|
Yellow to blue
|
Triturate 0.10g in 7.15 ml as above
|
4
|
Methyl red
|
4.2-6.3
|
Red to yellow
|
Use 18.60ml
|
5
|
Bromocresol purple
|
5.2-6.8
|
Yellow to blue-violet
|
Use 9.25 ml
|
6
|
Bromothymol blue
|
6.0-7.6
|
Yellow to blue
|
8.00ml
|
7
|
Phenol red
|
6.8-8.4
|
Yellow to red
|
14.20
|
8
|
Cresol red
|
7.2-8.8
|
Yellow to red
|
13.10
|
9
|
Thymol blue
|
8.0-9.6
|
Yellow to blue
|
10.75
|
10
|
Thymolphthalein
|
9.3-10.5
|
Colorless to blue
|
Dissolve 0.10g in 100ml rectified spirit
|
11
|
Thymol violet
|
9.0-13.0
|
Yellow to green to violet
|
Dissolve 0.01g of tropaeolin 0 in 100ml of distilled water. Dissolve 0.04g of thymolphthalein in a mixture of 50ml of rectified spirit and 50ml of water. Mix one part of tropaeolin O solution with 4 parts of thymolphthalein solution
|
Standard buffer solutions prepared as given below shall be kept in bottles made of alkali-free glass or of polyethene and shall not be used later than three months after preparation.
The solution from pH 1.2 to 2.2 shall be prepared by mixing 50ml of M/5 potassium chloride solution with the specified volumes of N/5 HCl as given below and diluting with distilled water to 200ml
pH
|
Volume in ml of N/5 HCl
|
1.2
|
64.5
|
1.4
|
41.5
|
1.6
|
26.3
|
1.8
|
16.6
|
2.0
|
10.6
|
2.2
|
6.7
|